*Corresponding Author:
Gian Marco Rosa,
Clinic of Cardiovascular Diseases, University of Genoa, Genoa, Italy, IRCCS Policlinico San Martino Genoa, Genoa, Italy
Tel: +39 3472738724
E-mail: Gian.Marco.Rosa@unige.it/gmrosa@libero.it
Abstract
Background and aim: During treatment with Angiotensin-Converting Enzyme Inhibitors (ACEIs), some dermatological adverse reactions may occur. The aim of this review was to focus on the main dermatological lesions which are associated with treatment with these medications.
Materials and methods: A search for the terms “angiotensin-converting enzyme inhibitors”, “dermatological effects”, “skin disease” was conducted in MEDLINE and PUBMED up to December 2019.
Results: A wide spectrum of dermatological lesions may occur in course of treatment with ACEIs, ranging from trivial forms to severe complications, including psoriasis, pemphigus and angioedema, the last being the most frequent dermatological side-effect.
Conclusion: Family doctors and clinicians should inform their patients of the increased risk of dermatological lesions associated with the use of ACEIs and instruct them to perform periodic self-examination of the skin.
Introduction
Worldwide, Angiotensin-Converting Enzyme Inhibitors (ACEIs) are very widely prescribed for their important cardiovascular effects. Binding to the active site of Angiotensin-Converting Enzyme (ACE), they block the conversion of Angiotensin I (AGT I) to Angiotensin II (AGT II), a peptide hormone that promotes peripheral vasoconstriction and unfavourable cardiac remodelling. Although they play a pivotal role in the management of a series of cardiovascular diseases-from arterial hypertension to heart failure-ACEIs may also cause several side effects involving multiple organs and apparatuses, including dermatological ones. Indeed, they may determine 2-3% of all adverse cutaneous drug reactions, according to an Italian study [1].
Angioedema
Angioedema implies non-inflammatory, non-pruritic and well-demarcated, non-pitting swelling that occurs in the form of large erythematous areas-typically involving lips, tongue, face, glottis, oropharynx, periorbital or perioral regions-with an onset over minutes to hours. It is similar to urticaria, but concerns deeper tissues (reticular dermis and hypodermis) and may have immunologic, non-immunologic, or idiopathic aetiology. It is usually a benign condition, but it can cause respiratory distress and death if severe laryngeal oedema occurs. The diagnosis is mostly clinical.
In case of ACEI-induced angioedema, Bradykinin (BK), a protein inducing vasodilatation and increasing endothelial permeability that is normally degraded by ACE, plays a major role in its pathogenesis. As shown in figure 1, ACEIs inhibit the degradation of BK in fragmentation products, thus leading to increased BK circulating levels and angioedema.

Figure 1: Mechanism of ACEI-induced angioedema.
The frequency of this side-effect during ACEI treatment is not negligible, ranging from 0.1% to 0.7% [2-4]. In a national medical chart abstraction study conducted by the United States Veterans Affairs Administration on nearly 600,000 patients, the incidence of angioedema after the initiation of ACEI therapy was 1.97/1,000 [5].
Angioedema is much more infrequent with Angiotensin II Receptor Blocker (ARB) than with ACEI administration, because ARBs antagonize AGT II receptors and are not directly responsible for increased BK levels in the blood.
Beyond ACEI withdrawal, there is no universal consensus on the pharmacological management of this dangerous adverse reaction. The most specific therapies are icatibant (a BK receptor antagonist) and C1-inhibitor concentrate, while tranexamic acid may be used for prophylaxis [6,7]. After regression of angioedema, switching to ARBs may be considered.
Psoriasis
Psoriasis is a common disease, affecting 2-3% of the population in Northern Europe. Beside a genetic susceptibility, possible risk factors are stress, bacterial and viral infections, smoking, obesity and alcohol and drug use. In a study of 1,203 patients affected by psoriasis, 23.2% were taking more than three systemic medications and 11.1% of these were taking more than 10 medications [8].
Although there are few data are available and no current studies confirm a causal relationship between the two, ACEI administration seem to possibly exacerbate rather than provoke psoriasis, which is considered an independent cardiovascular risk factor [9,10]. Since ACEIs are mainly used for cardiac diseases, this becomes an important concern.
The mechanism whereby ACEIs can induce or worsen psoriasis has not been completely clarified yet. Kinin-kallikrein system is probably involved, which could explain why stronger stimulants of kallikrein/ bradykinin pathway-e.g., ramipril-are more often associated with psoriasis [11]. For the same reason, AGT II receptor blockers appear to be less related to such kind of skin lesion [12]. Age might play a role too, and an analysis of case-controlled and case-crossover studies showed an association between ACEIs and psoriasis in patients older than 50 years [13]. Moreover, use of calcium-channel blockers or thiazides, often associated to ACEIs to better control arterial hypertension, could be a significant predictor of the development of psoriasis, according to one study [14].
Drug-induced psoriasis may be indistinguishable from idiopathic forms, thus constituting a diagnostic challenge for the physician. Its clinical manifestations can range from multiple plaques to severe erythroderma, as well as its severity can widely vary.
There is little evidence that any specific therapy proves efficacious, but drug withdrawal-together with topic steroids if required-is generally sufficient [15]. In case of fever and serious systemic involvement, intravenous methylprednisolone may turn out useful [11].
Pemphigus
The term “pemphigus” indicates a rare group of blistering autoimmune erosive and/or bullous diseases that affect the skin and mucous membranes. It is caused by antibodies directed against desmoglein-the principal protein forming desmosomes, i.e., the attachment points on the keratinocyte membrane-with subsequent loss of intercellular adhesion through a process called acantholysis [16]. The annual incidence of the two main variantspemphigus vulgaris and pemphigus foliaceus-is estimated at 0.5-3.2 cases per 100,000 in the general population, increasing in subjects of Ashkenazi Jewish descent and those of Mediterranean origin. The overall prevalence is calculated 100/1,000,000. Mortality of 5-15% has been reported [17].
Although uncommon, several drugs seem to be related to this disease, and pemphigoid manifestations following the administration of captopril, lisinopril, quinapril and enalapril have been described [1821]. Some of these molecules were demonstrated to unfavourably interact with the adhesion proteins on the keratinocyte surface through their thiol group, leading to acantholysis [20,22]. Other possible mechanisms are immunological, e.g., antigen modification or inhibition of suppressor T-cells.
When ACEIs with a thiol group are responsible for pemphigus, spontaneous recovery usually follows drug withdrawal. In contrast, spontaneous recovery is rare when ACEIs without a thiol group are implicated and administration of systemic steroids or cetirizine is often necessary [20,23,24].
Photosensitivity and Cancer
Photosensitive drug-reactions occur when sunlight exposure de- termines manifestations that are directly related to certain medica- tions, whose chemical structure allows the absorption of ultraviolet radiation [25]. They may be dose-dependent or not.
The prevalence of photosensitivity ranges from 0.5 to 8.9% of the general population [26].
Albeit ACEIs are not considered photosensitizing drugs, some cas- es have been reported during administration of ramipril, captopril, quinapril and enalapril [27-30].
Drug-induced photosensitivity may damage cellular DNA, thus promoting cutaneous carcinogenesis in the long term [31].
For what concerns treatment, drug withdrawal, protection from the sun, and topical steroids are usually efficacious [32].
The table 1 below resumes the role of ACEIs with respect to the cutaneous conditions that have been extensively treated in the text.
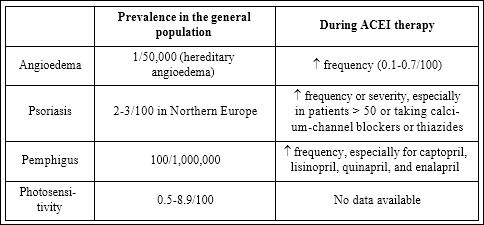
Table 1: ACEIs with respect to the cutaneous conditions that have been extensively treated in the text.
Conclusion
Although relatively infrequent, a large spectrum of dermatological side effects may occur during ACEI administration, representing a real clinical issue and sometimes requiring drug withdrawal. Angioedema constitutes a severe and potentially lethal mucocutaneous reaction; conversely, it is the most frequent side effect described after cough. In addition, ACEIs may induce or exacerbate psoriasis, more often in elderly patients. Finally, treatment with ACEIs may be associated with bollous disease, especially pemphigus vulgaris. As for cancer, the relation between cutaneous tumours and ACEIs remains weak, even though some of them may cause relevant photosensitization.
References
- Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, et (1999) Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol 48: 839-846.
- Sarkar P, Nicholson G, Hall G (2006) Brief review: Angiotensin converting enzyme inhibitors and angioedema: Anesthetic Can J Anesth 53: 994-1003.
- Kostis WJ, Shetty M, Chowdhury YS, Kostis JB (2018) ACE Inhibitor-Induced Angioedema: A review. Curr Hypertens Rep 20: 55.
- Bezalel S, Mahlab-Guri K, Asher I, Werner B, Sthoeger ZM (2015) Angiotensin-converting enzyme inhibitor-induced Am J Med 128: 120-125.
- Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, et al. (2008) Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension 51: 1624-1630.
- Sinert R, Levy P, Bernstein JA, Body R, Sivilotti MLA, et al. (2017) Randomized trial of icatibant for angiotensin-converting enzyme inhibitor-induced upper airway angioedema. J Allergy Clin Immunol Pract 5: 1402-1409.
- Beauchêne C, Martins-Héricher J, Denis D, Martin L, Maillard H (2018) Tranexamic acid as first-line emergency treatment for episodes of bradykinin-mediated angioedema induced by ACE inhibi Rev Med Interne 39: 772-776.
- Zahl V, Gerdes S, Mrowietz U (2005) Co-medication in patients with severe psoriasis: First results of a retrospective analysis in 1203 hospitalized patients in Germany. The 4th International Congress-The Royal College of Physicians, London, England.
- Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, et (2018) Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol 3: 949-956.
- Cohen AD, Bonneh DY, Reuveni H, Vardy DA, Naggan L, et al. (2005) Drug exposure and psoriasis vulgaris: Case-control and case-crossover studies. Acta Derm Venereol 85: 299-303.
- Thakor P, Padmanabhan M, Johnson A, Pararajasingam T, Thakor S, et al. (2010) Ramipril-induced generalized pustular psoriasis: Case report and literature review. Am J Ther 17: 92-95.
- Smallridge RC, Gamblin GT, Eil C (1986) Angiotensin-converting enzyme: Characteristics in human skin fibroblasts. Metabolism 35: 899-904.
- Katz M, Seidenbaum M, Weinrauch L (1987) Penicillin-induced generalized pustular J Am Acad Dermatol 17: 918- 920.
- Kim H-N, Han K, Song S-W, Lee JH (2018) Hypertension and risk of psoriasis incidence: An 11-year nationwide population-based cohort PLoS One 13: 0202854.
- Stavropoulos PG, Kostakis PG, Papakonstantinou AM, Panagiotopoulos A, Petridis AD (2003) Coexistence of psoriasis and pemphigus after enalapril intake. Dermatology 207: 336-337.
- Ohata C, Ishii N, Furumura M (2014) Locations of acantholysis in pemphigus vulgaris and pemphigus foliaceus. J Cutan Pathol Nov 41: 880-889.
- Ahmed AR, Moy R (1982) Death in J Am Acad Dermatol 7: 221-228.
- Marques Pinto G, Lamarao P, Vale T (1992) Captopril-induced pemphigus vegetans with Charcot-Leyden crystals. J Am Acad Dermatol 27: 281-284.
- Butt A, Burge SM (1995) Pemphigus vulgaris induced by captopril. Br J Dermatol 132: 315-316.
- Cozzani E, Rosa GM, Drosera M, Intra C, Barsotti A, et al. (2011) ACE inhibitors can induce circulating antibodies directed to antigens of the superficial epidermal Arch Dermatol Res 303: 327-332.
- Lo Schiavo A, Guerrera V, Cozzani E, Aurilia A, Ruocco E, et al. (1999) In vivo enalapril-induced acantholysis. Dermatology 198: 391-393.
- Wolf R, Tamir A, Brenner S (1991) Drug-induced versus drug-triggered pemphigus. Dermatologica1 82: 207-210.
- Palleria C, Bennardo L, Dastoli S, Iannone LF, Silvestri M, et al. (2018) Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers induced pemphigus: A case series and literature Dermatol Ther 20: 12748.
- Gornowicz-Porowska J, Dmochowski M, Pietkiewicz P, Bowszyc-Dmochowska M (2015) Mucosal-dominant pemphigus vulgaris in a captopril-taking woman with angioedema. An Bras Dermatol 90: 748-751.
- Su KA, Habel LA, Achacoso NS, Friedman GD, Asgari MM (2018) Photosensitizing antihypertensive drug use and risk of cutaneous squamous cell carcinoma. Br J Dermatol 179: 1088-1094.
- Verrotti A, Basciani F, Trotta D, Cutarella R, Salladini C, et (2002) Photoparoxysmal responses in non-epileptic children in long-term follow-up. Acta Neurol Scand 105: 400-402.
- Wagner SN, Welke F, Goos M (2000) Occupational UVA-induced allergic photodermatitis in a welder due to hydrochlorothiazide and Contact dermatitis 43: 245-246.
- Pérez-Ferriols A, Martínez-Menchón T, Fortea JM (2005) Follicular mucinosis secondary to captopril-induced photoallergy. Actas Dermosifiliogr 96:167-170.
- Rodríguez Granados MT, Abalde T, García Doval I, De la Torre C (2004) Systemic photosensitivity to quinapril. J Eur Acad Dermatol Venereol 18: 389-390.
- Kanwar AJ, Dhar S, Ghosh S (1993) Photosensitive lichenoid eruption due to enalapril. Dermatology 187: 80.
- Monteiro AF, Rato M, Martins C (2016) Drug-induced photosensitivity: Photoallergic and phototoxic reactions. Clin Dermatol 34: 571-581.
- Sánchez-Borges M, González-Aveledo LA (2011) Photoallergic reactions to angiotensin converting enzyme inhibitors. J Eur Acad Dermatol Venereol 25: 621-622.
Citation: Merlo AC, Rosa GM, Porto I (2020) Cutaneous Side Effects of Angioten- sin-Converting Enzyme Inhibitors. J Cardio Cardiovasu Med 4: 010.
Copyright: © 2020 Merlo AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


