*Corresponding Author:
Anatoly Langer,
Department of Medicine, Canadian Heart Research Centre, 259 Yorkland Road, North York, Ontario, Canada
Tel: +1 4169996264
E-mail: langera@CHRC.net
Abstract
Background: The objective of this report was to assess the care gap in the management of Atrial Fibrillation (AF) using a national chart audit. Care gap was defined as the difference between actual practice and that recommended by the Canadian Cardiovascular Society (CCS) guidelines.
Method/results: A total of 81 primary care physicians responded to an electronic survey detailing the care of 435 AF patients (age≥18 years who had no significant heart valve disorder). The median CHADS2 score was 2; the median HAS-BLED score was 2. Most (91.7%) had a CHADS2 of ≥1. The CHADS2 scores were estimated/guessed in 9.0% and 22.3% for HAS-BLED. In patients with a CHADS2≥1, 95.7% of patients were anticoagulated and 21 of the 36 patients with CHADS2 of 0 were anticoagulated (13 of the latter 36 patients were ≥ 65 years old). Warfarin was used in 26.0% of patients whereas novel oral anticoagulants (NOACs) were used in 66.7%. There was a tendency to favor warfarin over NOACs in patients with higher bleeding risk. Applying results from our study to the Canadian population up to 455 thromboembolic events, 1038 major bleeds and 525 intracranial hemorrhages could have been prevented annually had all AF patients been treated with NOACs.
Conclusion: Our study provides a contemporary portrait of management of AF in Canada. Although risk assessment tools remain underused, the vast majority of patients with thromboembolic risk warranting anticoagulation were receiving it. Nearly a quarter of the patients were not receiving the recommended first line treatment (NOACs).
Keywords
Atrial fibrillation; Oral anticoagulation
Abbreviations
AF: Atrial Fibrillation
CCS: Canadian Cardiovascular Society
eGFR: estimated Glomerular Filtration Rate
INR: International Normalized Ratio
NOAC: Novel Oral Anticoagulants
TTR: Time in Therapeutic Range
Introduction
Atrial Fibrillation (AF) is the most common sustained arrhythmia and affects 1-2% of the population [1,2]. The incidence of AF increases with age with an estimated 6% of the population age 65 years and over being afflicted [3]. The most feared outcome of AF is a thromboembolic event resulting in a fatal or debilitating stroke. The risk for thromboembolism can be estimated using a variety of risk based tools; the most commonly used one being CHADS2 (one point each for congestive heart failure, hypertension, Age >75 years, diabetes mellitus, and two points for prior Stroke/transient ischemic attack/non-central nervous system thrombo embolism). Since oral anticoagulation is frequently recommended, the risk of a thromboembolic event must be weighed against the probability of a major bleeding event; a consideration that should be discussed with all patients individually. The HASBLED score (hypertension, abnormal renal/liver function, stroke, bleeding history, labile International normalized ratio, elderly, drugs/ alcohol) is a tool often used to individualize estimates of bleeding risk for this purpose. Although current guidelines recommend using calculation tools to assess the risk of stroke and risk of hemorrhage, these tools are underused as evidenced in our previous publications [4-6].
The purposes of this report were to assess contemporary management for stroke prevention in patients with non valvular AF in Canada relative to the 2014 Canadian Cardiovascular Society AF guidelines and to identify care gaps in the management of anticoagulation in these patients.
Methods
Question AF (quality enhancement initiative to evaluate stroke risk and improve outcomes in patients with atrial fibrillation) was developed by the Canadian Heart Research Centre and the steering committee as an evidence based, maintenance of competence program accredited by the College of Family Physicians of Canada and the Fédération des Médecins Omnipraticiens du Québec, was approved by a central ethics board, and was funded through an educational support by the BMS-Pfizer Alliance.
The goals of this maintenance of competence program were to: 1) summarize contemporary practice in AF management by Canadian family physicians; 2) provide practice based support tools and address self identified needs and practice identified care gaps. Approximately 850 primary care physicians across Canada were invited to participate.
Participating physicians were asked to submit data for 10 consecutive non valvular AF patients from their practice. This database of information was used to generate this report. Physicians were not compensated for participation.
Patients of age ≥18 years with documented paroxysmal, permanent or persistent AF who did not have a significant heart valve disorder (i.e., prosthetic valve, hemodynamically significant or severe valve disease as assessed by the clinician), clinically significant hepatic disease (e.g., active hepatitis), or a reversible cause of AF (e.g., recent cardiac surgery, pulmonary embolus and untreated hyperthyroidism) were eligible for participation. Information collected for each patient included demographics, AF history, medical history, stroke risk assessment and method by which it was determined (including, but not restricted to, CHADS2 and CHA2DS2-VASc [congestive heart failure, hypertension, Age over 75 years, diabetes mellitus, stroke, vascular disease, Age 65-74 years, sex category]), bleeding risk assessment and method by which it was determined (including, but not restricted to, HAS-BLED ), current antithrombotic therapy, and most recent INR values (if on warfarin). This information was gathered by means of an electronic chart audit form created and maintained by the Canadian Heart Research Centre.
Descriptive analyses of demographic variables, risk score calculations, and other variables pertinent to AF management were performed. Continuous variables were summarized as a mean and standard deviation and discrete variables were reported as counts and percentages. Calculations were performed to determine the estimated stroke and bleeding risk, using the CHADS2, CHA2DS2-VASc, and HAS-BLED indices. Using up to 6 of the most recently available INR values, estimates of the time in the Therapeutic Range (TTR) were established using the Rosendaal method, with the therapeutic range defined as an INR range between 2.0 and 3.0. All analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
A total of 435 patients (60% male) who were 74.4±10.9 years old were recruited by 81 physicians from all provinces from February to October 2014 (Table 1). The average duration of AF was 5.6±5.0 years with permanent AF being most common (38%) followed by persistent AF (31%) and paroxysmal AF (29%); in 2% the AF had not been classified. Prior history of stroke was present in 11%, TIA in 10%, and non neurologic systemic embolism in 2%. Prior rhythm management included cardio version in 9%, ablation in 5%, and 73% were on anti arrhythmic medication. The most common co morbidities were hypertension (72%), smoking (39%), coronary artery disease (34%), diabetes mellitus (30%), congestive heart failure (20%) and peripheral arterial disease (9%). Prior history of major bleeding was documented in 5% of patients, liver disease in 2%, stage 3 chronic renal disease (eGFR 30-59ml/min) in 38%, stage 4 chronic renal disease (eGFR <30 ml/min) in 5%, and heavy alcohol consumption (>10 drinks/week) in 2.5%. Review of current medications revealed 59% use of beta blockers, 55% use of lipid lowering therapy, 32% use of an angiotensin converting enzyme inhibitor, 23% use of an angiotensin receptor blocker, 29% use of a diuretic, 15% use of a calcium channel blocker, 5% use of amiodarone, and 5% use of sotalol.
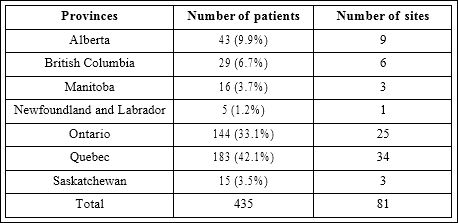
Table 1: Distribution of Patients by Provinces.
The thromboembolic risk based on CHADS₂ score was 6 in 2% of the patients, 5 in 3% of the patients, 4 in 8% of the patients, 3 in 23% of the patients, 2 in 31% of the patients, 1 in 25% of the patients, and 0 in 8% of the patients. In contrast, physicians’ estimate of the CHADS2 score was 0 in 16% of the patients. Guesses or estimates were used by physicians in 9% of the CHADS2 scores and 22% of HAS-BLED scores. We also calculated the CHA2DS2-VASc score which was 0 in 3% , 1 in 7%, 2 in 14%, 3 in 20%, 4 in 26%, 5 in 14%, 6 in 9%, 7 in 5%, 8 in 2% and 9 in 1 % (median score was 4).
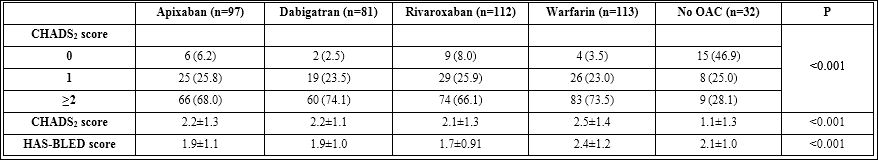
Table 2: Comparison with and without anticoagulant therapy groups.
Note: Comparing among groups with anticoagulant therapy, p value=0.59, 0.19 and <0.001 for the above CHADS2 score group 0, 1≥2; CHADS2 score and HAS-BLED score respectively.
Therapy According to Thromboembolic Risk
Among 435 patients, 113 (26.0%) were anticoagulated using warfarin whereas 66.7% of patients were on NOACs: apixaban (22.3%), dabigatran (18.6%) and rivaroxaban (25.8%). Further details regarding the use of specific oral anticoagulant therapies are shown in table 2.
Of the 399 patients with a CHADS₂ score ≥1, 382 patients (95.7%) were on anticoagulant therapy as per the CCS AF guideline recommendations. Of the 17 patients with a CHADS₂ score ≥1 who were not anticoagulated the CHADS₂ score was 1 in 2 patients, 2 in 4 patients, 3 in 2 patients, 4 in 4 patients, 5 in 4 patients and 6 in 1 patient. Among these 17 patients the HAS-BLED score was 1 in 1 patient, 2 in 7 patients, 3 in 5 patients and 4 in 4 patients. Of these 17 patients, 11 patients received ASA, 1 patient received clopidogrel, and 5 received no antithrombotic therapy (1 refused, 3 had history of prior bleed and 1 had high bleeding risk).
Among the 382 patients on anticoagulant therapy, 109 (29%) were on warfarin. Two or more International Normalized Ration (INR) measurements were available for 102 of these 109 patients (94%) allowing calculation of a Time in Therapeutic Range (TTR). The average TTR was 72±29%. The TTR was ≥70% in 63% of the patients. Comparison of the two most populous provinces revealed a better INR management in Quebec sites as compared to sites in Ontario : TTR 84±21% vs 59±30% (p=0.0005).
Of the 36 patients with a CHADS₂ score of 0, 21 patients (58%) received anticoagulant therapy; 13 of the 21 (62%) were age 65 or older which is consistent with the current recommendation for anticoagulation. Using the prior set of guidelines from 2012 [7], which were in operation when these data were compiled, anticoagulation would have been recommended for 17 of these 21 patients who were women, had history of vascular disease or were older than 65 years of age. Thus, there remained 4 patients with CHADS₂ and CHA2DS2-VASc score of zero for whom no reason could be found to justify the use of anticoagulation.
The average HAS-BLED score of patients receiving warfarin was greater than that of patients receiving one of the newer direct oral anticoagulants (p<0.001; Table 2). In 43 patients with a HAS-BLED score >3, 19 patients (44%) received one of the newer direct oral anticoagulants, 20 patients (47%) received warfarin, and 4 patients (9%) received neither.
The use of specific newer direct oral anticoagulants and their dosages as a function of renal clearance is shown in table 3 and these were mostly consistent with the product monographs. None of the patient with an estimated Glomerular Filtration Rate (eGFR) below 15 mL/min received a NOAC, but some patients with an eGFR below 30mL/min did. For apixaban, where a combination of two out of three characteristics (age ≥80 years, weight ≤60 kg, creatinine >133 µmol/L) warrants a lower dosage, no patient received a dosage over that which was recommended.
The Impact of the Care Gap in Stroke Prevention
Given that the CCS recommends NOACs over warfarin based on data from previous studies, we expanded the results from our study to the Canadian population to assess the potential effects of this care gap [8-10].
We assumed that there were 350,000 patients with non valvular AF based on the estimated prevalence of 1% in the general population [11]. We further assumed that those on warfarin (29% or 101 500 patients) with CHADS₂ scores ≥1 would benefit to a greater degree from treatment with one of the newer direct oral anticoagulants as recommended by the CCS AF guidelines [4]. Using the average CHADS₂ score from our study of 2 (translating into a 4% annual risk of thromboembolic events) and using a 3.3% annual risk of major bleeds and 0.75% annual risk of intra cranial bleeds, we calculated the annual frequency of these events in these 101 500 Canadian non valvular AF patients [8-10]. With these assumptions we estimate that if patients were not treated with an anticoagulant there would have been 4060 strokes each year related to non valvular AF, which would have been reduced to 1339 strokes (a 67% relative reduction) if these patients were treated with warfarin [12]. The 101 500 patients treated with warfarin would experience 3349 major bleeding events per year of which 761 would be intracranial bleeds. If the CCS recommendations were followed and one the newer direct oral anticoagulants were used rather than warfarin, the results of RE-LY, ROCKET-AF and ARISTOTLE would predict that up to 455 strokes, 1038 major bleeds, and 525 intracranial hemorrhages could be prevented each year in Canada (Table 4) [8-10].
Discussion
The results of this study show that, in Canada, patients with an indication for anticoagulation therapy in the setting of non valvular atrial fibrillation are very likely to be receiving such therapy. Nevertheless, the choice of anticoagulation agent was less consistent with current recommendations [4].
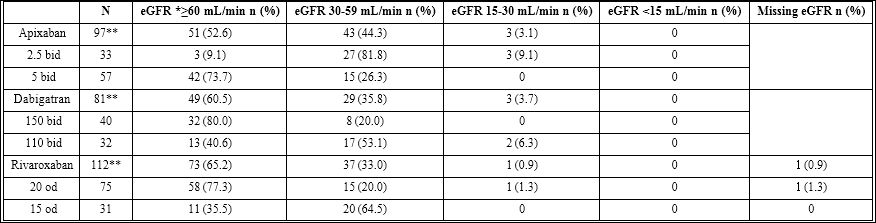
Table 3: Therapy according to renal clearance.
eGFR: estimated Glomerular Filtration Rate.
**These numbers do not represent the total of the different dosages since some dosages were unavailable.
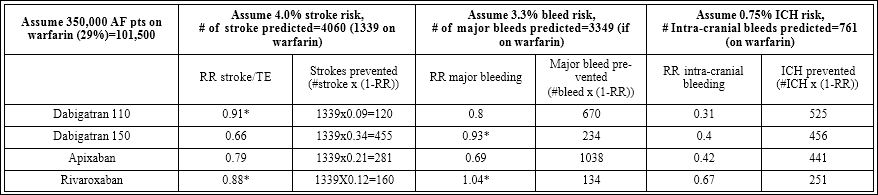
Table 4: Benefits of novel anticoagulants.
*Not statistically significant
We demonstrated an excellent anticoagulation rate of 95.7% in patients with a CHADS2 score ≥1 which clearly demonstrates that Canadian physicians are following the guidelines and have incorporated a risk based approach to treatment into their practice. Although this result is consistent with those of the recent connect-AF study, it suggests improvement over those of earlier studies and studies in other jurisdictions [6]. A 2007 publication from a Seattle based database reported 75% compliance to antithrombotic guidelines and a 2013 Garfield registry study reported that only 60% of patients warranting anticoagulation were receiving such therapy [13,14]. Of note, the 2014 update of the CCS AF guidelines was used to analyze the data we collected from February to October 2014. Hence, much of the data collection was accomplished prior to their publication in October 2014. The main change in recommendations was in patients under the age of 65 with a CHADS2 score of 0: in 2012, anticoagulation was recommended for a female with vascular disease who was under the age of 65 whereas, in 2014, anticoagulation was no longer recommended for this group. Overall, treatment decisions observed in this dataset were consistent with both the 2012 and 2014 CCS guidelines.
The tendency to underestimate the CHADS2 score was still present as in our previous study with 16% of patients having a score of 0 when estimated by the physician compared to 8% when formally calculated [6]. The physicians guessed or estimated CHADS2 scores in 9% of patients and HAS-BLED scores in 22% of patients which is concordant, even improved, compared to prior data [6]. Regardless, using the calculated thromboembolic risk score and the current guidelines, the vast majority of patients requiring anticoagulation were receiving it. When Canadian physicians use warfarin, they achieve time in the therapeutic range averages that exceed those generally reported with a mean TTR of 72%±29% [15,16]. However, almost a quarter of the non valvular AF patients requiring anticoagulation were not treated according to the CCS AF guidelines in that warfarin was chosen over novel anticoagulants. Furthermore, the use of warfarin was greatest in patients at highest risk for bleeding suggesting a misconception about the safety profile of warfarin.
The novel anticoagulants such as dabigatran, rivaroxaban and apixaban have been proven to be safe and effective alternatives to warfarin in the RE-LY, ROCKET-AF and ARISTOTLE studies respectively [8-10]. These trials demonstrated that apixaban and dabigatran 150 mg have superior efficacy over warfarin for prevention of stroke and systemic embolism. Apixaban and dabigatran 110 mg have been shown to reduce major hemorrhage compared with warfarin and all the newer direct oral anticoagulants have been shown to reduce intracranial hemorrhage compared to warfarin. A recent meta analysis including these three pivotal studies demonstrated statistically significant reductions in the risks of stroke or systemic thromboembolism, in intracranial bleeds, and in all cause mortality [16]. Based on such data, considered high quality evidence, the CCS AF guidelines strongly recommend the use of one of the newer direct oral anticoagulants over warfarin therapy. Nevertheless, 29% of the patients who required anticoagulation according to the guidelines were anticoagulated with warfarin. The care gap in the optimal use of the newer direct oral anticoagulants in this setting was further exaggerated by an even greater warfarin use in patients with higher HAS-BLED scores. Analysis in the present study found no apparent medical reason for the choice of warfarin over one of the newer direct oral anticoagulants such as reduced renal function. Undoubtedly, cost considerations constitute the major barrier to use of the newer direct oral anticoagulants since the Canadian provincial public insurance carriers do not cover the first line use of one of the newer direct oral anticoagulants which are reimbursed only if anticoagulation with warfarin is inadequate or contraindicated or if monitoring is not possible [17]. Thus, provincial cost containment schemes may not be in the best interests of the patients.
Potential reasons for choice of specific anticoagulant agents were not assessed in the present study. In the ORBIT-AF study, patients for whom dabigatran was chosen over warfarin were more likely to be younger, to be Caucasian, to be on a private insurance plan, to have new onset atrial fibrillation and to have a lower risk of thromboembolic events and of bleeding [18]. One of the most common incentives identified for switching patients from warfarin to dabigatran was specific patient request with higher education being a prominent factor.
Limitations
The design of our data set acquisition could not exclude the bias of physician selection, patient selection, or the Hawthorne effect. While the number of physicians participating was relatively small, we were able to detect important qualitative trends including the overall use of anticoagulant therapy in relation to guidelines and available risk stratification tools for stroke and bleeding risk. Physicians were not compensated for participation which may have limited the number of physician and therefore patient participants. We limited the scope of data acquisition to only those variables that are identified in the guidelines (4) as important for risk stratification and management decision making.
Conclusion
The present study provides insight into the real-world application of guidelines. It demonstrates a 95% rate of anticoagulation in non valvular AF patients with an indication for anticoagulation according to the Canadian Cardiovascular Society AF guideline (4). This trend suggests an improvement over previously reported compliance rates. However, almost a third of the patients in the present study received warfarin rather than one of the newer direct oral anticoagulants even though use of these newer agents is associated with fewer strokes, fewer major hemorrhages, and fewer intracranial hemorrhages. We hypothesize that cost considerations and a misconception regarding increased bleeding risk with the newer direct oral anticoagulants may have played a role. This care gap or treatment inertia may result in the occurrence of as many as 455 extra strokes, 1038 extra major bleeds, and 525 extra intracranial hemorrhages each year in Canada if our findings were extrapolated to the total Canadian AF population. Further studies to assess the reasons for this care gap would facilitate efforts directed towards improving patient care and are needed given the limitations of our observations. Management of AF patients that is more consistent with the Canadian guidelines (i.e., closing the care gap) will likely result in improved outcomes.
Funding Source
Role of the funding source: Question-AF was funded through an educational support from the BMS-Pfizer Alliance. Question AF was conceived, designed, coordinated and managed independently by the Canadian Heart Research Centre (CHRC). Question AF is supported by the BMS-Pfizer Alliance. The authors/steering committee had exclusive involvement in the collection, analysis and interpretation of data; in the writing of, and in the decision to submit, the manuscript.
Disclosure
A Langer: C - Clinical Trials; Company/Organization; Actelion, Amgen, Astra-Zaneca, Bayer, Boehringer-INgelheim, Bristol-Myers Squibb/ PFIZER Alliance, Sanofi, Servier, Valeant, I. Nguyen-Tri: None.
M. Tan: None.
B. Coutu: A - Consulting Fees/Honoraria/Salary; Company/Organization; Bayer. C - Clinical Trials; Company/Organization; BMS Pfizer, Bayer, BI, Sorin, St-Jude, Madtronic, Gilead, Biotronik
A. Duong: Employee of Bristol-Myers Squibb
J. Grégoire: A - Consulting Fees/Honoraria/Salary; Company/Organization; Amgen, AZ, Bayer, BI, BMS, Merck, Pfizer, Sanofi, Servier, Valeant
J. Habert: A - Consulting Fees/Honoraria/Salary; Company/Organization; Pfizer, BMS, BI, Astra Zeneca, Bayer, Amgen, NovoNordisk, Lilly.
L. Brent Mitchell: A - Consulting Fees/Honoraria/Salary; Company/ Organization; Bayer, Boehringer-Ingelheim, BMS, Pfizer, Medtronic Inc, St. Jude Medical. C - Clinical Trials; Company/Organization; Boehringer-Ingelheim.
D. Ngui: A - Consulting Fees/Honoraria/Salary; Company/Organization; Amgen, Astra Zeneca, BMS, Boeringer, Eli Lilly, Janssen, Merck, Pfizer, Novonordisk, Novartis, Valeant, Lundbeck, Takeda. B - Officer, Director, Or In Any Other Fiduciary Role; Company/Organization; Health Choices First.
D. Paquette : None
Z.A. Wulffhart: A - Consulting Fees/Honoraria/Salary; Company/Organization; Boerhinger Ingelheim, Pfizer, Bristol Meyers Squibb, Bay- er, Servier. C - Clinical Trials; Company/Organization; Boerhinger, St Jude Medical, Medtronic, Biosense.
References
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, et al. (20010) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) JAMA 285: 2370-2375.
- Stewart S, Hart C, Hole D, McMurray J (2001) Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86: 516-521.
- Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, et al. (1997) American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Risk factors. Stroke 28: 1507-1517.
- Verma A, Cairns JA, Mitchell LB, Macle L, Stiell IG, et al. (2014) 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial Can J Cardiol 30: 1114-1130.
- Goodman S, Kerr C, Green M, Gladstone DJ, Mendelsohn A, et al. (2001) 171 The risk stratification and stroke prevention therapy care gap in canadian atrial fibrillation patients: Insights from the Facilitating Review and Education to Optimize Stroke Prevention in Atrial Fibrillation (FREEDOM AF) knowledge translation program. Canadian Journal of Cardiology 27: 121.
- Patel AD, Tan MK, Angaran P, Bell AD, Berall M, et al. (2015) Risk stratification and stroke prevention therapy care gaps in Canadian atrial fibrillation patients (from the Co-ordinated National Network to Engage Physicians in the Care and Treatment of Patients With Atrial Fibrillation chart audit). Am J Cardiol 115 : 641-646.
- Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, et al. (2012) Focused 2012 Update of the Canadian Cardiovascular Society Atrial Fibrillation Guidelines: Recommendations for Stroke Prevention and Rate/Rhythm Con Can J Cardiol 2012;28:125-136.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139-1151.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883-891.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, et (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365: 981-992.
- Heart and Stroke (2015) Statistics. Heart and Stroke Foundation, USA. at: http://www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3483991/k.34A8/Statistics.htm#atrialfib.
- Hart RG, Pearce LA, Aguilar MI (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146: 857-867.
- Glazer NL, Dublin S, Smith NL (2007) Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med 167: 246-252.
- Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, et al. (2013) Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD PLoS One 8: 63479.
- Wells G, Coyle D, Steiner S, Coyle S, Kelly AT, et al. (2012) Safety, effectiveness, and cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada.
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, et al. (2014) Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised tri The Lancet 383: 955-962.
- http://www.hc-sc.gc.ca/hcs-sss/pharma/acces/ptprog-eng.php.
- Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, et al. (2013) Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibril J Am Heart Assoc 2: 000535.
Citation: Langer A, Tan M, Mitchell LB, Habert J, Coutu B, et al. (2017) Contemporary Trends in Canada for Stroke Prevention in Atrial Fibrillation: Quality Enhancement Initiative to Evaluate Stroke Risk and Improve Outcomes in Patients with Atrial Fibrillation (Question AF). J Cardio Cardiovasu Med 2: 005.
Copyright: © 2017 Langer A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


