*Corresponding Author:
Anatoly Langer,
Canadian Heart Research Centre, 259 Yorkland Road, North York, Ontario, Canada
Tel: +1 4169996264
E-mail: langera@CHRC.net
Abstract
Background: The objective of this study was to assess the care gap in the management of risk factors among high cardiovascular risk patients with type 2 Diabetes Mellitus (DM) who were not at guidelines recommended A1C and LDL-C target despite first line therapy.
Methods: A total of 82 primary care physicians enrolled 586 patients with DM, high risk based on Framingham score, LDL-C > 2.0 mmol/L despite optimal statin therapy, and A1C > 7.0% despite metformin therapy. Physicians were asked to follow patients on three occasions: baseline and 4-12 weeks and 18-26 weeks, and received reminders to optimize therapy for lipid and glycemic control to achieve guideline recommended targets.
Results: Among enrolled patients 57% were male, average age was 62.7±10.5 years, and 95% had 10-year Framingham risk score of CV event ≥20%; prior history of cardiovascular diagnosis was present in 20%, smoking history in 38%, blood pressure > 130/80 mmHg in 68%. At baseline, the lipid profile in mmol/L was: total cholesterol 4.88±1.04, LDL-C 2.82±0.75, HDL-C 1.25±0.45 and non HDL-C 3.63±1.07, triglycerides 1.93±1.05. Glycemic profile included fasting plasma glucose of 8.35±2.01mmo/L and A1C 7.94±0.75%. At baseline medications for dyslipidemia management were statin alone in 75% of patients and statin plus another lipid lowering therapy in 25%, and for glycemic control were metformin alone in 71% and metformin in fixed dose combination in 29%. During the follow up LDL-C declined significantly (p< 0.0001) to 2.28±0.87 (visit 2) and 2.17±0.92 (visit 3) while proportion of patients achieving LDL-C ≤ 2.0 mmol/L (co-primary end-point) was zero at baseline and 43% and 50% respectively during the second and third visits. The A1C declined to 7.58±1.18 and 7.53±1.30 respectively and proportion of patients achieving the A1C target (co-primary end-point) increased from zero at baseline to 35% and 43% respectively for visits two and three (p < 0.0001). With respect to blood pressure target achievement (< 130/80), it was 32% at baseline, 45% at visit 2 (4-12 weeks) and 47% at visit 3 (18-26 weeks) (p=0.0047). All three targets (BP, A1C and LDL-C) were achieved in 7% at visit 2 and 14% at visit 3.
Conclusion: The present study provides insight into the real-world application of guidelines and the need to overcome treatment inertia. It demonstrates that among patients with DM who have not yet achieved control of dysglycemia and dyslipidemia, the use of clinical reminder maybe of help in improving management.
Introduction
Clinical Practice Guidelines (CPGs) from professional organizations in Canada such as Canadian Diabetes Association (CDA) and Canadian Cardiovascular Society (CCS) advocate that patients with type 2 Diabetes Mellitus (DM) should have their risk factors managed in an aggressive and timely manner [1,2]. These recommendations are largely based on seminal type 2 diabetes-focused trials demonstrating significant improvements in vascular complications and reduced mortality through comprehensive and multifactorial behavioral modification and pharmacotherapy strategies [3,4]. However, despite concerted and widespread efforts to translate these evidence-based recommendations into routine clinical practice as well as increasing pharmacologic options, practice reviews conducted in Canada indicate that optimal management of type 2 diabetes patients remains challenging [5].
We have previously documented that the use of clinical reminders may be helpful in achieving recommended targets, though the success in following the guidelines can be variable [6,7]. The purposes of this medical practice activity was to 1) describe a Canadian patient population with DM that is not achieving recommended targets for LDL-C and A1C; 2) to study whether simple clinical reminders may improve target achievement and 3) to report challenges as perceived by physicians in following the evidence-based recommendations.
Methods
Cholestabetes was developed by the Canadian Heart Research Centre (CHRC) as an evidence-based, medical practice activity. The authors (AL and LAL) developed patient chart audit forms, educational tools and assisted with the provision of relevant resources for use by participating physicians. The program was supported by Valeant Canada which funded CHRC at arm’s length to engage 100 Canadian primary care physicians and to enroll 750 patients. Invitations were sent to primary care physicians across Canada inviting them to participate. The invitation was distributed through e-mail and facsimiles by CHRC to lists of Canadian primary care physicians, including those who were participants in prior or ongoing registries within the CHRC. Physicians were reimbursed for their time involvement. The program was reviewed and approved by Optimum clinical research, an independent central ethics review board. All aspects of the program deployment, including data capture using eCRF were coordinated by CHRC and the ownership of all data resided with CHRC. Patient enrolment started in November 2014 and ended in May 2016 with data entry for follow up completed in February 2017. Inclusion criteria were patients older than 18 years of age with diagnosis of primary hypercholesterolemia and DM, high risk for cardiovascular disease (defined as one of: 10-year risk of cardiovascular event ≥20% based on the Framingham risk score, prior diagnosis of coronary artery disease, cerebrovascular disease, abdominal aortic aneurysm surgery, or peripheral arterial disease and LDL > 2.0 mmol/L despite optimal statin therapy (defined as atorvastatin ≥ 20 mg, rosuvastatin ≥ 10 mg, simvastatin or pravastatin ≥ 40 mg) as well as A1C > 7.0% and < 9.0% on metformin therapy as well as consent to participate [1]. The exclusion criteria were clinically significant concomitant illness or co-morbid condition (e.g., cancer), liver, muscle or kidney abnormalities (e.g., compromises patient management according to physician), secondary causes of hypercholesterolemia (e.g., hypothyroidism, nephrotic syndrome) and contraindications or intolerance to combination therapy. Patients were seen on three occasions (baseline and two more clinically driven visits at 4-12 weeks and 18-26 weeks) for follow up in management of their dyslipidemia and dysglycemia.
The eCRF developed and deployed by CHRC allowed gathering of clinically relevant variables and had an interactive clinical reminder step during which physicians were reminded of four possible add-ons for lowering of the LDL-C (ezetimibe, Bile Acid Sequestrant (BAS), fibrate or niacin) according to the CDA recommendations and were also reminded that according to the CDA CPG a particular BAS, colesevelam, also has A1C lowering effects, although it does not have that indication in Canada [1].
The co-primary outcome of the MPA was proportion of patients achieving LDL-C and A1C target. Descriptive analyses of demographic variables were performed. Continuous variables were summarized as a mean and standard deviation and discrete variables were reported as counts and percentages. Changes in risk factors during follow up were compared using Cochran-Armitage Trend test or Cochran’s Q test. All analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
A total of 586 patients (57% male) who were 62.7±10.5 years old were recruited by 82 physicians from all provinces except PEI (Ontario 66%, Quebec 13%, BC 5%, Manitoba 7%, 9% almost equally from New Brunswick, Newfoundland, Saskatchewan, and Nova Scotia) and who were 60% Caucasian, 16% south-east Asian, 11% southAsian, 8% black, and 1% each Hispanic, Arabic/North African, Aboriginal Canadian, multi-racial, and other.
The baseline characteristics are summarized in table 1.
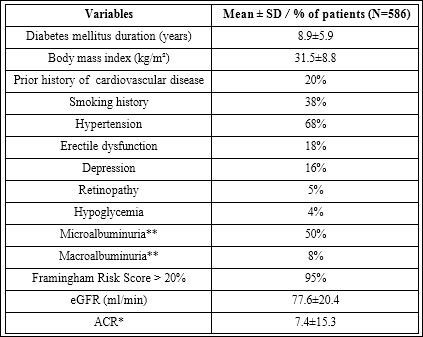
Table 1: Baseline characteristics.
* Normal: < 2.0 for men, <2.8 for women
** Micro (2.0-20.0 for men, 2.8-28.0 for women) and macro (>20 for men, >28.0 for women)
Medications at baseline for dyslipidemia management were statin alone in 74% of patients, statin with another lipid lowering drug in 22% (ezetimibe 11%, colesevelam 5% and fibrate 3%, omega-3 fatty acids in 3% and cholestyramine in 1% and niacin in 0.2%). Statin and two other lipid lowering drugs were used in 3% (ezetimibe and colesevelam in 2% and ezetimibe and fibrate in 1%). Among patients on statin therapy, rosuvastatin was used in 54%, atorvastatin in 42%, and simvastatin in 4% of patients.
At baseline medications for glycemic control were metformin alone in 71% and metformin in fixed dose combination in 29% (metformin/sitagliptin in 18%, metformin/saxagliptin in 6%, metformin/ linagliptin in 3%, metformin/sitagliptin XR in 2%). Overall, at baseline 28% were on metformin monotherapy, 39% were on two treatments, 26% on three and 6% on four and above.
The other current medications of interest were ACE inhibitor or ARB in 76%, ASA or another antipletelet agent in 44%, diuretic in 26%, calcium channel blocker in 25% and beta blocker in 22%.
The impact of clinical reminders on risk factor control
At baseline, the lipid profile was: total cholesterol 4.88±1.04, LDL-C 2.82±0.75, HDL-C 1.25±0.45, non-HDL-C 3.63±1.07 and tri glycerides 1.93±1.05 mmol/L. The LDL-C declined significantly (p< 0.0001) to 2.28±0.87 during visit 2 and 2.17±0.92 during the final visit 3. The proportion of patients achieving the LDL-C ≤ 2.0 mmol/L (co-primary end-point) was zero at baseline and 43% and 50% respectively during the second and third visits. These changes in lipid lowering were achieved after visit 1 with addition of colesevelam in 48% of patients, ezetimibe in 11% and fibrate in less than 1% and no change in 41% and after visit 2 (if LDL-C was still above 2.0 mmol/L) with the addition of colesevelam in 23%, ezetimibe in 10% and no change in 67%.
When asked why no additional therapy was prescribed despite LDL-C above target, the following reasons were listed: reinforcing lifestyle change in 30%, more time needed to evaluate efficacy of current therapy in 28%, recent up titration of the statin dose 16%, patient refusal 12%, belief that current management is appropriate 8%, medication cost in 4%, and medical constraints such as co-morbid conditions or contraindications in 2%.
At baseline patients on colesevelam had similar LDL-C to those on ezetimibe (3.05±0.69 vs 3.03±0.91 mmol/L, p=0.89), however at the end of the third visit their LDL was lower (1.97±0.79 vs 2.38±1.04 mmol/L respectively, p=0.046). At baseline glycemic profile was fasting plasma glucose of 8.35±2.01mmo/L and A1C 7.94±0.75%. During follow up the fasting glucose declined to 7.80±2.00 on visit 2 and to 7.69±2.02 on visit 3. The A1C declined to 7.58±1.18 and 7.53±1.30 respectively and proportion of patients achieving the A1C target (co-primary end-point) increased from zero at baseline to 35% and 43% respectively for visits two and three (p < 0.0001). These improvements in glycemic control were achieved after visit 1 with addition of other antihyperglycemic therapy in 32% (SGLT2i in 11%, DPP4i in 9%, sulfonylurea in 5%, insulin in 4%, GLP1 receptor agonist in 2%, and 1% together for alpha-glucosidase inhibitor and meglitinide). Importantly no change was undertaken in 68%, however in those with addition of colesevelam for lipid lowering, there was a recommendation to not yet add other antihyperglycemic therapy at the same time because of the dual action of the colesevelam on both LDL-C and A1C lowering.
When asked why no additional therapy was prescribed despite A1C above target, the following reasons were listed: awaiting potential benefit of colesevelam treatment in 40%, need more time to evaluate efficacy of current treatment in 23%, reinforcing lifestyle advice in 20%, current treatment is appropriate in 8%, patient refusal in 7% and the cost in 2%.
Overall, patients treated with colesevelam had similar A1C as those not treated with it (7.46±1.07 vs 7.64±1.16, p=0.11) and the number of other antihyperglycemic medications did not differ significantly between these two groups (2.5 ±1.1 with colesevelam vs 2.3±1.0 without p=0.09).
With respect to blood pressure target achievement (< 130/80), it was 32% at baseline, 45% at visit 2 and 47% at visit 3 (p=0.0047). All three targets (BP, A1C, and LDL-C) were achieved in 7% at visit 2 and 14% at visit 3 (Figure 1).
Discussion
The results of Cholestabetes MPA demonstrate that many of the high cardiovascular risk patients with DM who are not at recom- mended A1C and LDL-C target despite first line lipid and glycemic control therapy can be provided with a clinical reminder which results in almost half of these patients achieving the recommended target (Figure 1).
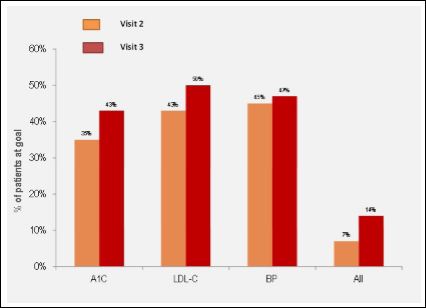
Figure 1: Proportion of patients achieving guidelines recommended targets during the follow up.
We have previously demonstrated a consistent care gap of 40-50% in the risk factor management among patients with DM which we de- fined as a proportion of patients that do not achieve a recommend- ed target [6,8,9]. The achievement of the so-called triple target (BP, LDL-C and A1C) varied between 13 and 20% [8,9]. The Cholestabetes program studied whether a clinical reminder may be of help to clini- cians in closing the care gap and increasing the target achievement.
Our findings indicate that clinical reminder maybe of help as part of an overall approach towards optimization of care and overcoming clinical inertia [10]. We also found that clinicians were responsive to nuances of recommendations as exemplified by the choice of coleseve- lam as identified in the CDA guidelines as being useful in lowering LDL-C as well as A1C. We have previously demonstrated that clinical reminder for LDL-C lowering can result in 50% reduction in the care gap and our findings from the current experience support this and ex- tend them to the glycemic control [11]. What was also noteworthy was that while there was no specific attempt to focus on blood pressure control, participating physicians were aware of its importance and there was consistent increase in the proportion of patients achieving target blood pressure based on published recommendations. The low- ering of the LDL-C supported by the clinical reminder and achieved by whatever means is worthwhile achievement in these higher risk group of patients, regardless of what class of agents recommended by the guidelines were used to achieve it [12]. Similarly, there was a significant decrease in A1C during follow up. The specific impact of colesevelam on lowering of the A1C was not discernible and did not lead to an overall reduction in the number of glycemic control thera- pies, although the A1C reduction overall supports the usefulness and practical applicability of the clinical reminder.
It is important to note that the choice for LDL-C lowering did not include the use of a Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) inhibitor such as alirocumab or evolocumab that are now available in Canada but were not at the time of baseline enrolment of our program. We believe the use of clinical reminder based on peer-reviewed guidelines may be of even greater help to clinicians as the number and complexity of choices and the need for combination therapy increases. Moreover, since many of the patients with diabetes in primary care, including those with cardiovascular disease, may not have their LDL-C optimally controlled, the use of clinical reminder maybe of help in supporting the guidelines and evidence based rec- ommendations [5,6].
Limitations
The design of our data set acquisition could not exclude the bias of physician selection, patient selection, or the Hawthorne effect (the modification of behavior of the participating physicians due to their awareness of being monitored). Lack of a control group does not allow for a definitive conclusion regarding the efficacy of a clinical reminder. It also remains unclear whether increase in the proportion of patients achieving the target was a result of additional therapy as part of the routine care or greater patient adherence or both. The evidence for additional therapy is the strongest for LDL-C control because of the documented increase in the use of colesevelam and ezetimibe and the weakest for blood pressure control since it was not in focus but exhibited a similar trend towards improvement.
Conclusion
The present study provides insight into the real-world appli- cation of guidelines and the need to overcome treatment inertia. It demonstrates that among patients with DM who have not yet achieved control of dysglycemia and dyslipidemia, the use of clinical reminder maybe of help in improving management.
Funding Source
Role of the funding source: Cholestabetes was funded through an educational support from the Valeant Canada.
Disclosure
A Langer has received research and educational grant support from Valeant Canada as well as from Actelion, Amgen, Astra Zeneca, Bayer, BI, BMS, Merck, Servier and Sanofi.
M Tan: None
L Goldin: None
D Ngui: has received research funding from, has provided CME on behalf of, and/or has acted as an adviser to Valeant ,Amgen, Lund- beck, Merck, Mylan, Astra Zeneca, BMS, BI, Lilly, Lundbeck and Novo Nordisk
Vincent Woo: None
L A Leiter: has received research funding from, has provided CME on behalf of, and/or has acted as an adviser to AstraZeneca, Amgen, Boehringer Ingelheim, Eli Lilly, Esperion, GSK, Janssen, Kowa, The Medicines Company, Merck, Novo Nordisk, Sanofi and Servier
References
- Canadian Diabetes Association Clinical Practice Guidelines Expert Commit- tee, Cheng AY (2013) Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Intro- duction. Can J Diabetes 37: 1-3.
- Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, et al. (2016) 2016 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in the Can J Cardiol 32: 1263-1282.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589.
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O (2008) Effect of a multifactorial intervention on mortality in type 2 N Engl J Med 358: 580-591.
- Leiter LA, Berard L, Bowering CK, Cheng AY, Dawson KG (2013) Type 2 diabetes mellitus management in Canada: Is it improving? Can J Diabetes 37: 82-89.
- Katz PM, Mendelsohn AA, Goodman SG, Langer A, Teoh H (2011) Use of a Treatment Optimization Algorithm Involving Statin-Ezetimibe Combination Aids in Achievement of Guideline-Based Low-Density Lipoprotein Targets in Patients With Dyslipidemia at High Vascular Risk Guideline-Based Under- taking to Improve Dyslipidemia Management in Canada (GUIDANC). Can J Cardiol 27: 138-145.
- McLaughlin VV, Langer A, Tan M, Clements PJ, Oudiz RJ, et al. (2013) for the PAH QuERI Investigators CHEST 143: 324-332.
- Braga M, Casanova A, Teoh H, Dawson KC, Gerstein HC, et (2010) Treat- ment Gaps in the Management of Cardiovascular Risk Factors in Patients with Type 2 Diabetes in Canada. Can J Cardiol 26: 297-302.
- Braga MF, Casanova A, Teoh H, Gerstein HC, Fitchett DH (2012) Poor achievement of guidelines-recommended targets in type 2 diabetes: Findings from a contemporary prospective cohort study. Int J Clin Pract 66: 457:464.
- Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, et (2001) Clinical inertia. Ann Intern Med 135: 825–834.
- Katz PM, Mendelsohn AA, Goodman SG, Langer A, Teoh H, et al. (2011) Use of a Treatment Optimization Algorithm Involving Statin-Ezetimibe Com- bination Aids in Achievement of Guideline-Based Low-Density Lipoprotein Targets in Patients With Dyslipidemia at High Vascular Risk
- Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, et al. (2016) Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Me- ta-analysis. JAMA 316: 1289-1297.
Citation: Langer A, Tan M, Goldin L, Ngui D, Woo V (2017) Cholestabetes Medical Practice Activity (MPA): Overcoming Treatment Inertia in Diabetes. J Cardio Cardiovasu Med 2: 007.
Copyright: © 2017 Langer A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.


